ABOUT THE CHEMICAL REACTIONS LESSON
- Chemical Reactions Unit Miss E. Mac's Classification
- Chemical Reactions Unit Miss E. Mac's Classic
- Chemical Reactions Unit Miss E. Mac's Classics
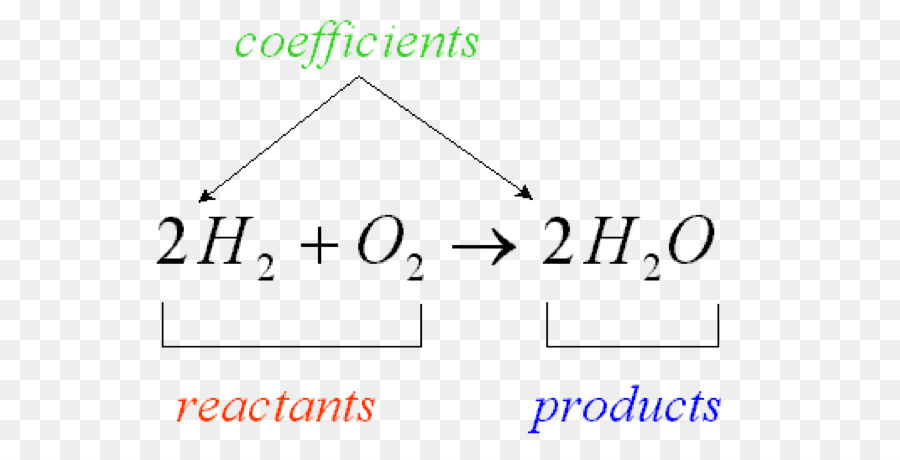
Chem I Unit 5 Reactions Chemical Reaction: a process in which one or more substances are converted into new substances with different physical and chemical properties Reactant: a substance that enters into a chemical reaction Product: A substance produces by a chemical reaction. Chemical Changes- The second grade students will investigate some chemical changes and reactions and should be able to identify at least four characteristics that indicate a chemical change. Chemical Changes - To learn that the formation of gas bubbles is an indication of a chemical change. The half-life of a chemical reaction is the time required for the concentration of the reactants to reach half of their initial value. For first-order reactions, the relationship between the reaction half-life and the reaction rate constant is given by the expression: t 1/2 = 0.693/k. ABOUT THE CHEMICAL REACTIONS LESSON. This lesson is appropriate for children ages 11 and up. Students are introduced to atoms, molecules, compounds, and mixtures, using LEGO® bricks as atoms. In an engaging hands-on wet lab, students experience a chemical reaction and then model the same reaction with LEGO bricks. Reactants are the starting substances of a reaction. Products are the substances formed during the reaction. Representing Chemical Reactions As chemists, we can describe chemical reactions in different ways. In a sentence 2. Word equations 3. Skeleton equation 4. Chemical equations reactant 1 + reactant 2 product 1 + product 2.
This lesson is appropriate for children ages 11 and up. Students are introduced to atoms, molecules, compounds, and mixtures, using LEGO® bricks as atoms. In an engaging hands-on wet lab, students experience a chemical reaction and then model the same reaction with LEGO bricks. This lesson is also offered as a 3-hour academic field trip LEGO Chemistry at the Edgerton Center.
Download the Molecule Sets Overview or read the Rationale for using LEGO bricks as atoms.
MOLECULE SET MATERIALS
We are no longer able to sell Molecule Sets, unfortunately. Please visit our Information for Edgerton Center Molecule Sets webpage that describes how to put your own set together.
The following LEGO bricks are the minimum required (per kit/2 students) for the Chemical Reactions Lesson:
- 6 red 2x4
- 2 pink 2x4
- 2 light green 2x4
- 2 black 2x4
- 1 green 2x4
- 4 white 1x2
Chemical Reactions Mats (per kit/2 students):
Chemical Reactions Unit Miss E. Mac's Classification
Student Worksheets:
TEACHING THE CHEMICAL REACTIONS LESSON
Co-Teaching Video from BLOSSOMS:
Teacher Guides and Resources:
MIT Edgerton Molecule videos on our YouTube channel:
- Chemical Reactions Wet Lab Video (7.5 min)
CURRICULUM STANDARDS
This lesson meets the following items of the Massachusetts State Frameworks for grades 6-8, Physical Sciences Strand: Elements, Compounds & Mixtures:
5. Recognize that there are more than 100 elements that combine in a multitude of ways to produce compounds that make up all of the living and nonliving things that we encounter.
6. Differentiate between an atom (the smallest unit of an element that maintains the characteristics of that element) and a molecule (the smallest unit of a compound that maintains the characteristics of that compound).
7. Give basic examples of elements and compounds.
8. Differentiate between mixtures and pure substances.
10. Differentiate between physical changes and chemical changes.
ADDITIONAL RESOURCES
Document Versions prior to June 2012:
LEGO®, the LEGO logo, and the brick and knob configurations are trademarks of the LEGO group, used here with permission. ©MIT. All rights reserved.
Chemical Reactions Unit Miss E. Mac's Classic
ABOUT THE CHEMICAL REACTIONS LESSON
This lesson is appropriate for children ages 11 and up. Students are introduced to atoms, molecules, compounds, and mixtures, using LEGO® bricks as atoms. In an engaging hands-on wet lab, students experience a chemical reaction and then model the same reaction with LEGO bricks. This lesson is also offered as a 3-hour academic field trip LEGO Chemistry at the Edgerton Center.
Download the Molecule Sets Overview or read the Rationale for using LEGO bricks as atoms.
MOLECULE SET MATERIALS
We are no longer able to sell Molecule Sets, unfortunately. Please visit our Information for Edgerton Center Molecule Sets webpage that describes how to put your own set together.
The following LEGO bricks are the minimum required (per kit/2 students) for the Chemical Reactions Lesson:
- 6 red 2x4
- 2 pink 2x4
- 2 light green 2x4
- 2 black 2x4
- 1 green 2x4
- 4 white 1x2
Chemical Reactions Mats (per kit/2 students):
Student Worksheets:
TEACHING THE CHEMICAL REACTIONS LESSON
Co-Teaching Video from BLOSSOMS:
Teacher Guides and Resources:
MIT Edgerton Molecule videos on our YouTube channel:
- Chemical Reactions Wet Lab Video (7.5 min)
Chemical Reactions Unit Miss E. Mac's Classics
CURRICULUM STANDARDS
This lesson meets the following items of the Massachusetts State Frameworks for grades 6-8, Physical Sciences Strand: Elements, Compounds & Mixtures:
5. Recognize that there are more than 100 elements that combine in a multitude of ways to produce compounds that make up all of the living and nonliving things that we encounter.
6. Differentiate between an atom (the smallest unit of an element that maintains the characteristics of that element) and a molecule (the smallest unit of a compound that maintains the characteristics of that compound).
7. Give basic examples of elements and compounds.
8. Differentiate between mixtures and pure substances.
10. Differentiate between physical changes and chemical changes.
ADDITIONAL RESOURCES
Document Versions prior to June 2012:
LEGO®, the LEGO logo, and the brick and knob configurations are trademarks of the LEGO group, used here with permission. ©MIT. All rights reserved.
